Research team of Dr. Wei Chi and Dr. Youjin Hu from Zhongshan Ophthalmic Center discovered novel molecular biomarkers in monocyte subsets for VKH disease
Source: Zhongshan Ophthalmic Center
Written by: Zhongshan Ophthalmic Center
Edited by: Tan Rongyu, Wang Dongmei
Vogt–Koyanagi–Harada (VKH) disease is one of the common sight-threatening uveitis entities in the Asian population and carries a high risk of blindness. It is a systemic refractory autoimmune disease, affecting multiple organs and characterized by bilateral granulomatous panuveitis and systemic disorders, including leukoderma, paratrichosis, and various central nervous system and auditory signs. While the clinical features and diagnostic criteria are well described, the pathogenesis of VKH disease remains unclear. Moreover, there are currently no reliable disease-specific biomarkers to objectively and accurately evaluate the in vivo immune status at different disease stages and predict the response to treatment.
Recently, a research team directed by Dr. Wei Chi and Dr. Youjin Hu from Zhongshan Ophthalmic Center discovered a pathogenic subpopulation of monocyte that was activated in VKH and ISG15 can serve as a biomarker for the diagnosis of VKH syndrome. The research paper entitled "Genetic landscape and autoimmunity of monocyte in developing Vogt-Koyanagi-Harada disease" was published online on September 28, 2020, as a research article in
PNAS.
The study identified six populations with two previously unreported subsets according to similarities and differences in transcriptome profiles: 1) The S100A12 monocytes represented the commonalities among CD14high monocytes and expressed hallmark proinflammatory. 2) HLA monocytes expressed the MHC Class II molecules LGALS2 and CPVL. 3) CD16 monocytes were a nonclassical population. 4) Proinflammatory monocytes presented a unique combination of genes, including chemokine ligands, interleukins, and nod-like receptors associated with virus infection, the inflammatory process, and pyroptotic cell death mediators. 5) Megakaryocyte-like monocytes expressed genes similar to megakaryocyte progenitors (including PPBP and PF4). 6) NK cell-like monocytes expressed a cytotoxic gene signature and shared other differentially expressed genes with NK cells.
Fig. 1. Clustering analysis of blood monocytes and cell population identification.
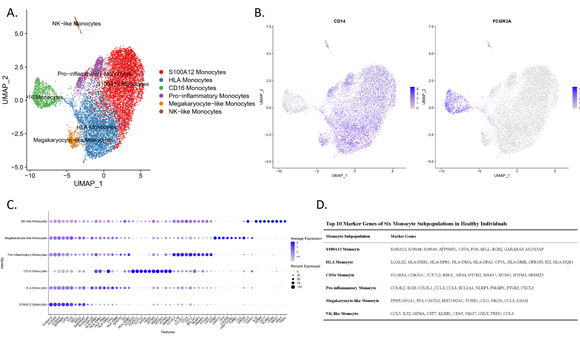
Fig. 2. Single-cell profiling of human circulating monocytes.
Among the subpopulations, proinflammatory monocytes demonstrated the greatest gene expression changes in the acute stage of VKH disease, suggesting that phenotypic alterations of this subpopulation may be related to VKH disease development.
Fig. 3. Mapping monocyte atlas of VKH patients compared with the healthy controls.
Although clinical measures can monitor disease activity and treatment response, such as fluorescein fundus angiography (FFA) and optical coherence tomography (OCT), these assessments yield little information on pathogenic processes, immune status, or the mechanisms underlying treatment responses. To identify a blood-based index of VKH disease, they examined IFN-stimulated gene 15 (ISG15), which specifically highly expressed in the proinflammatory monocytes, and found that ISG15 expression was up-regulated predominantly in the monocyte from VKH patients and the level of ISG15 in VKH patients was normal after 3 mouths treatment, suggested production of ISG15 might monitor disease activity and therapeutic response.
Fig 4. The production of ISG15 in VKH patients was reduced after immunosuppressive therapy.
Dr. Youjin Hu, Ph.D. student Yixin Hu and M.S. student Yuhua Xiao are the first authors. Dr. Wei Chi is the corresponding author. This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and grants from the Science and Technology Program of Guangzhou.
Link to the paper:
https://www.pnas.org/content/early/2020/09/25/2002476117
