Research advances in the immobilization of functional biomacromolecules from Prof. Gangfeng Ouyang’s group at School of Chemistry
Source: School of Chemistry
Written by: School of Chemistry
Edited by: Wang Dongmei
Metal–organic-frameworks (MOFs), made by linking inorganic and organic units with strong bonds (reticular synthesis), have emerged as promising platforms for diverse applications, including gas storage, catalysis, and separation. Owing to their finely tunable chemical composition, morphology, and robust structure, a very recent advanced application in MOF chemistry was the use of MOFs as protective networks for immobilizing functional biomacromolecules, for example, DNA and proteins, including enzymes, in the fields of nanomachines, biosensing, and biocatalysis. This immobilization offers effective control over the reaction process and enhanced biomacromolecule stability in storage and operational conditions. However, biomacromolecules are susceptible to degradation and denaturation, and thus current immobilization strategies are usually carried out by impregnating the biomacromolecules into pre-synthesized materials with large pore dimensions. This comes with substantial challenges, including low loading efficiency, incompact confinement, and the restricted pore size.
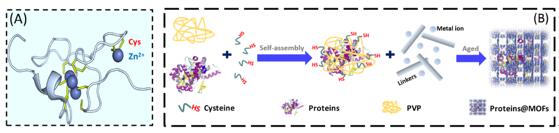
Figure 1. (A) Illustration of the Zn2+-binding by metallothionein. This Zn2+-binding is due to the formation of mercaptide bonds; (B) Schematic representation of the amino-acid-boosted one-pot embedding (AAOPE) strategy.
Recently, Prof. Gangfeng Ouyang’s group at School of Chemistry, Sun Yat-sen University, published a research paper entitled “A Convenient and Versatile Amino-Acid-Boosted Biomimetic Strategy for the Nondestructive Encapsulation of Biomacromolecules within Metal–Organic Frameworks” in
Angew. Chem. Int. Ed. (IF=12.102), a renowned chemistry journal in the world. This work proposed an amino-acid-boosted one-pot embedding (AAOPE) strategy that enabled the rapid encapsulation/co-encapsulation of a broad range of proteins, including enzymes, within microporous MOFs. The key principle relied on accelerating the formation of prenucleation clusters around proteins, in the form of protein/polyvinylpyrrolidone (PVP)/cysteine (Cys) self-assembly. The inspiration for this stemmed from the metal cation accumulating pattern of the metallothioneins (Figure 1A). These proteins with abundant Cys accumulated Zn2+ions via mercaptide bond formation, thereby accelerating the formation of prenucleation clusters and the production of MOFs around the proteins (Figure 1B). In addition, The encapsulated proteins maintained their native conformations, and the structural confinement within porous MOFs endowed enzymes with excellent bioactivity, even in harsh conditions (e.g. in the presence of proteolytic or chemical agents or at high temperature). Furthermore, the feasibility of this biomimetic strategy for biostorage, enzyme cascades, and biosensing was also verified. It is believed that this convenient and versatile encapsulation strategy has great promise in the important fields of biomedicine, catalysis, and biosensing.
This work was supported by the National Natural Science Foundation of China (21225731, 21477166, 21527813, 21677182 and 21737006).
Link to the paper:
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201813060
