Prof. Liang-Hu Qu’s group at School of Life Sciences revealed an important role of non-coding RNA microRNA-122 in hepatocyte antiviral natural immunity
Source: School of Life Sciences
Written by: School of Life Sciences
Edited by: Wang Dongmei
Recently, Prof. Liang-Hu Qu’s group at School of Life Sciences, Sun Yat-sen University, published a research paper entitled "MicroRNA-122 supports robust innate immunity in hepatocytes by targeting the RTKs/STAT3 signaling pathway” in eLife, a renowned scientific journal for the biomedical and life sciences. This paper for the first time reported the important role of liver-specific small non-coding RNA microRNA-122 in hepatocyte antiviral innate immunity, and revealed that microRNA-122 confers robust innate immune function to hepatocytes by targeting the RTKs/STAT3 signaling pathway. The School of Life Sciences at Sun Yat-sen University is the only author unit. Hui Xu (teacher) and Shi-Jun Xu (PhD student) at School of Life Sciences are the first authors of this paper. Prof. Liang-Hu Qu is the corresponding author.
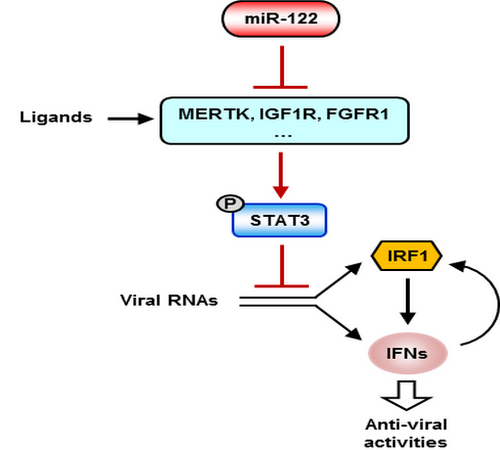
Figure 1. Illustration of the mechanism by which miR-122 regulates STAT3 phosphorylation and the IFN response
Intrinsic innate immune responses belong to a natural immune defense function that is possessed by every cell type in our body, which play critical roles in the defense against pathogens such as viruses. Because intrinsic innate immune responses is the basis of all immune responses, it is known as the first line of immune defense. The generation and regulation of innate immunity in different types of cells, particularly epithelial cells, is currently unclear. microRNA-122 is a small non-coding RNA molecule that is specifically and abundantly expressed in hepatocytes, but is significantly down-regulated or silenced in hepatocellular carcinoma cells. Prof. Qu’s group found that introduction of microRNA-122 into HepG2 cells can significantly enhance the innate immune responses of cells to various viral nucleic acids, including HCV and HBV. They found that microRNA-122 can directly target multiple receptor tyrosine kinases (RTKs), thereby reducing the level of tyrosine phosphorylation of STAT3 in HepG2 cells. More interestingly, they found that STAT3 directly inhibits the expression of the interferon regulatory factor IRF1, and that downregulation of STAT3 phosphorylation by microRNA-122 removes the repression of STAT3 on the trancriptional activation of interferons, allowing a rapid and strong activation of the interferon signaling in response to pathogen invasion. This study reveals an important role for the regulation of interferon expression by miR-122–RTKs/STAT3–IRF1–IFNs circuitry in hepatocytes, and elucidates the new mechanism by which microRNAs regulate the innate immunity of non-immune cells such as hepatocytes.
The expression of microRNA-122 is extremely abundant in mature hepatocytes, so hepatocytes can rapidly initiate immune defense in response to viral attacks. This strong innate immunity activity is essential for hepatocytes. Once the presence of microRNA-122 in hepatocytes is insufficient (for example, inflammation caused by viral infection can lead to down-regulation of microRNA-122 expression), it will lead to a significant decline in the innate immunity activity of hepatocytes, thereby more susceptible to virus invasion. These findings have important implications for the treatment of liver diseases caused by chronic infection of the virus, especially for liver cancers.
Link to the paper: https://elifesciences.org/articles/41159
