Professor Suhua Li from School of Chemistry Made Novel Polymer Material with Multidimensional Click Chemistry
Source: School of Chemistry
Edited by: Zheng Longfei, Wang Dongmei
The Click Chemistry, founded as a concept for creating functional molecular assemblies through highly efficient chemical bond formation by Nobel Laureate Professor Sharpless of Scripps Research in 1998 (217th ACS National Meeting, Anaheim, CA), is a fast and modular style of synthesis. Its goal is to accelerate the discovery of useful new substances or to create more functions. Two click reactions were subsequently developed by Professor Sharpless and co-workers that enable the formation of stable connections with unparalleled efficiency, namely: the Cu(I)-catalyzed azide–alkyne cycloaddition reaction (CuAAC) in 2002, and the Sulfur Fluoride Exchange (SuFEx) reaction in 2014. Over the years, CuAAC and SuFEx have made perspective-changing impact in many areas, including drug discovery, bioconjugation, and material science. The click “linkages” created via CuAAC- derived triazoles and the SuFEx connective gas SO
2F
2, are still confined to trajectories in a plane, while multidimensional connections will provide more potential applications.
Professor Suhua Li from the School of Chemistry at Sun Yat-sen University developed a safe and convenient procedure to make SOF
4 gas on basis of DuPont’s early recipe when he was a postdoctor in Sharpless Lab. Then, SOF
4 gas, which had been forgotten for almost half a century, could be easily produced in 100 grams scale one batch in the lab. With SOF
4, he worked with Professor Sharpless to develop Multidimensional Click Chemistry, implementing the fast and efficient assembling of amine-amine, amine-phenol, amine-phenol-phenol, and amine-phenol-amine. Due to the chiral configuration of sulfur, these substituents create 3-dimensional covalent departure vectors from the tetrahedral sulfur(VI) hub, and it is the first polyvalent click reaction that opens the door to another dimension and trajectory (
Angew. Chem. Int. Ed. 2017, 56, 2903). In the cooperation with Kelly Lab of the Scripps Research, they found that sulfuramidimidoyl fluorides, the product from the reaction of SOF
4 with primary and secondary amines sequentially, could target specific proteins with high activity and site selectivity in human cell lysate, and forming covalent linkage with specific amino acid side chains. Several important disease-related proteins were targeted in human cell such as poly(ADP-ribose) polymerase 1 (PARP1), macrophage migration inhibitory factor (MIF), soluble epoxide hydrolase (EPHX2) etc. (
Nature Chem. 2020, 12, 906).
On this basis, a collaboration of chemists that includes Professor Suhua Li, Professor Sharpless, Professor John Moses (Cold Spring Harbor Laboratory), Professor Han Zuilhof (Wageningen University), Professor Peng Wu (Scripps Research), and Director Yi Liu (Lawrence Berkeley National Laboratory), Professor Jianmei Lu (Soochow University) synthesized a novel polymer from SOF
4 (Figure 1). One feature of this polymerizaiton reaction is that there are two S–F bonds on each side of the iminosulfur oxydifluoride monomer. After one S–F bond is reacted during the polymerization process, the remaining one will be less active. Therefore, one S–F bond can be reacted with high selectivity, avoiding uncontrollable cross-linking reaction. The remaining S–F bond can also be reacted with aryl silyl ether further with a more efficient BEMP catalyst, or with a secondary amine in the presence of a base if needed to form S–O and S–N bonds respectively, thereby facilitating the preparation of well-distributed and highly branched polymers. Various functional molecules can be easily installed on this polymer and to form diversified functional polymers quickly. NMR experiment showed that N=SOF
2 group in the end position of oligomer increased significantly at the beginning of polymerization, and gradually decreased after reaching the maximum, demonstrating that the reaction was step-growth polymerization mechanism. At the same time, the N=SOF
2 group can be completely transformed within 200 seconds in the polymerization, showing the high efficiency of this reaction.
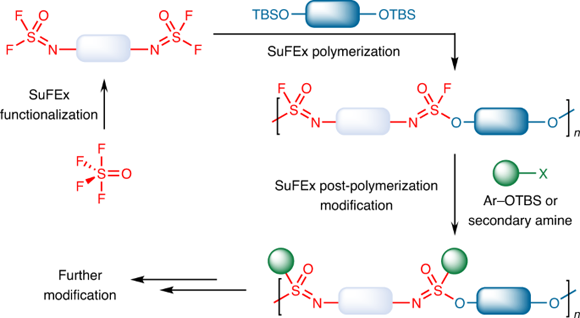
Figure 1. Novel Polymer from SOF
4 (www.nature.com)
This paper was published in
Nature Chemistry with the title "SuFExable polymers with helical structures derived from thionyl tetrafluoride". Professor Li is the first author, and the co-corresponding authors includes Professor Li, Professor Wu, Professor Zuilhof, Professor Moses, and Professor Sharpless. This work was supported by the National Natural Science Foundation of China, the Natural Science Foundation of Guangdong Province, the Program for Guangdong Introducing Innovative and Entrepreneurial Teams, and the Pearl River Talent Recruitment Program and many other funding. Dr. Jiajia Dong (Research Professor of the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences) provided helpful suggestions for the development of multi-dimensional click chemistry at the beginning of the project.
With the cooperation of several countries and research teams, SOF
4-based Multidimensional Click Chemistry has shown great potential applications in bioconjugation, drug development, and new materials. The Royal Society of Chemistry (RSC) awarded the Horizon Prize 2021 to the multidimensional click chemistry team led by Professor Sharpless (https://www.rsc.org/prizes-funding/prizes/2021-winners/multidimensional-click -chemistry-team/#undefined).
Link to the paper:
https://www.nature.com/articles/s41557-021-00726-x
