Professor Zong-Wan Mao and Associate Professor Cai-Ping Tan’ Group made important progress in the use of metal complexes as epigenetic regulators
Source: School of Chemistry
Edited by: Tan Rongyu, Wang Dongmei
Epigenetic modifications mainly include post-translational histone modification, DNA modification and RNA modification, which can affect gene expression by regulating transcription and chromatin structure. Demethylases of histone and RNA are also emerging as promising anticancer targets. Epigenetic regulation is multifaceted with modifications of histone, DNA and RNA acting coordinately, and modulating them concurrently may achieve a synergistic anticancer effect. However, co-regulation of these modifications presents a great challenge as valid inhibitors/activators are not available for many of the regulatory proteins. Therefore, it is a huge challenge to exert the synergy of these modifications. Recent studies have found that interference with mitochondrial metabolism and iron homeostasis is a very promising anti-cancer strategy. The intermediate products in mitochondrial metabolism are cofactors or natural inhibitors of histone/DNA/RNA demethylases, iron is the active center of biological macromolecule Fe(II)/2-oxoglutarate-dependent demethylation.
Recently, Professor Zong-Wan Mao and Associate Professor Cai-Ping Tan’s group designed three mitochondrial-targeted rhenium(I) complexes incorporating a clinical iron chelating agent, in order to break the mitochondrial metabolism and iron homeostasis simultaneously (Figure 1A). Among them, DFX-Re3 can relocate cellular iron to mitochondria and interfere with mitochondrial metabolism including the key metabolites related to epigenetic modifications (Figure 1B), and shows highly selective killing of triple-negative breast cancer effect. Moreover, relocating of iron causes the down-regulation of the Fe(II)/2-oxoglutarate-dependent demethylases. As a consequence, DFX-Re3 can elevate the levels of DNA, RNA and histone methylation simultaneously, which eventually leads to alternations in RNA polymerase II activities and gene expression profiles. Mechanism studies have shown that DFX-Re3 can induce cellular immunogenic apoptosis, and exhibits prominent antitumor activity in vivo. In all, our research provides a novel strategy to regulate cancer epigenome by intervening mitochondrial metabolism and iron homeostasis.
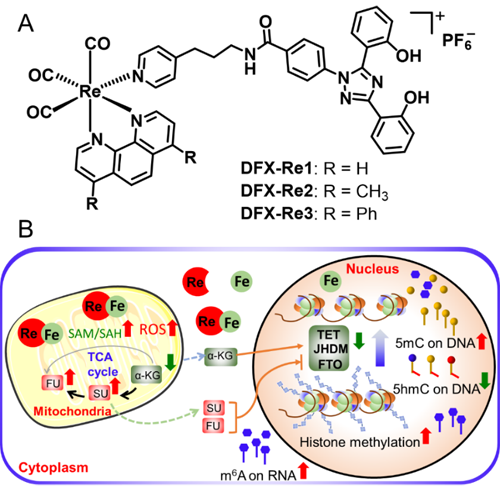
Research on the mechanism of action found that the design has the following significant advantages: (1) DFX-Re3 affects metabolites related to epigenetic modification in mitochondrial metabolism; (2) DFX-Re3 enriches cellular iron in mitochondria and induces mitochondria produce a large amount of reactive oxygen species; (3) DFX-Re3 simultaneously increases the methylation levels of histones, DNA and RNA; (4) DFX-Re3 changes the transcriptome, especially the activity of RNA polymerase II; (5) DFX-Re3 induces cellular immunogenic apoptosis and shows high anti-cancer activity in vivo.
Recently this research data was published as a very important paper (VIP) in
Angewandte Chemie International Edition. The paper is entitled “Recoding Cancer Epigenome by Intervening Metabolism and Iron Homeostasis with Mitochondria-Targeted Re(I) Complexes”. Professor Zong-Wan Mao (School of Chemistry, Sun Yat-sen University) and Associate Professor Cai-Ping Tan (School of Chemistry, Sun Yat-sen University) are the co-corresponding authors. PhD candidate Zheng-Yin Pan (School of Chemistry, Sun Yat-sen University) is the first author.
This work was supported by the National Natural Science Foundation of China (nos.21778078, 91953117 and 21837006), the innovative team of Ministry of Education (no.IRT_17R111), the Guangdong Natural Science Foundation (2015A030306023), and the Fundamental Research Funds for the Central Universities.
Link to the paper:
https://doi.org/10.1002/anie.202008624
