Research Advances in Dormancy of Bone Metastatic Cancer Cells from Study Group of Professor Xinsheng Peng
Source: The First Affiliated Hospital
Written by: The First Affiliated Hospital
Edited by: Wang Dongmei
The research article entitled “Wnt5a induces and maintains prostate cancer cells dormancy in bone” by the study group of Prof. Xinsheng Peng from Department of Spine Surgery, the First Affiliated Hospital of Sun Yat-sen University cooperating with the study group of Prof. Libing Song from Department of Experimental Research, State Key Laboratory of Oncology in Southern China, Sun Yat-sen University Cancer Center is published online in international prestigious journal Journal of Experimental Medicine (IF=10.79) on December, 28th, 2018. The first author affiliation is the First Affiliated Hospital of Sun Yat-sen University, the correspondence authors are Prof. Xinsheng Peng and Prof. Libing Song, and the first authors are Dr. Dong Ren, Dr. Yuhu Dai and Dr. Qing Yang from the study group of Prof. Xinsheng Peng.
Prostate cancer (PCa) is the second most common malignancy in male worldwide. The principal characteristic of PCa is its high propensity to metastasize to bone, which is the leading cause responsible for PCa-associated death. In a substantial fraction of PCa patients, metastatic bone tumors can appear years and even decades later, following excision of primary PCa. This clinical phenomenon is generally explained by the finding that PCa cells disseminate to bone marrow before any treatment and enter into a dormant state in the osteoblastic niche. However, the critical mechanisms inducing and maintaining the disseminated PCa cells in the osteoblastic niche remain to be further clarified.
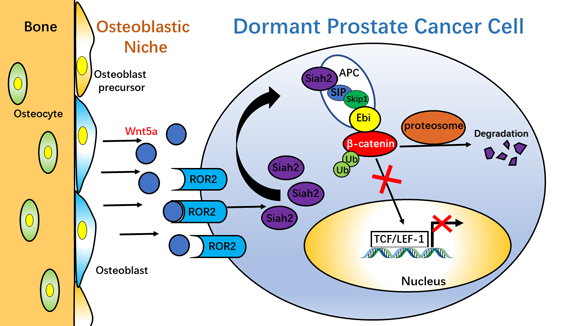
Schematic model for Wnt5aROR2SIAH2-induced dormancy of PCa cells in bone
The findings from the study group of Prof. Xinsheng Peng have demonstrated that Wnt5a from osteoblastic niche induces and maintains dormancy of PCa cells in bone via activating non-canonical ROR2/SIAH2 signaling that represses Wnt/β-catenin signaling (schematic model below), which is one of the pivotal mechanisms contributing to the dormancy of disseminated PCa cells in the osteoblastic niche. The important scientific issue that whether activation of non-canonical Wnt signaling-induced inhibition of canonical Wnt signaling induces and maintains dormancy of cancer cells is fully explained by the findings in the current study. The results from animal experiments in vivo reveal that recombinant Wnt5a maintains the dormancy of disseminated PCa cells in bone and prevents the formation of metastatic bone tumor via circumventing the re-activation of PCa cells, suggesting a potential therapeutic utility of Wnt5a via inducing dormancy of PCa cells in bone. This work was supported by grants from the National Natural Science Foundation of China (no. 81472505).