Probiotic roles of Clostridium butyricum (C.B) are involved in regulating disease and cancers, yet the mechanistic basis for these regulatory roles remains largely unknown. C.B. has been found to play an important role in colorectal cancer (CRC) treatment, according to a recent study “Therapeutic potential of Clostridium butyricum anticancer effects in colorectal cancer” published in Gut Microbes by Professor Mong-Hong Lee's team at the Institute of Gastroenterology, The Sixth Affiliated Hospital, Sun Yat-sen University.
CRC is one of the deadliest cancer types with strong resistance to various therapies and is associated with dysbiosis of microbiota. Various studies have explored associations between microbiota and CRC; however, the molecular mechanisms behind these associations are not well characterized. The roles of probiotic bacteria C.B in CRC regulation are not known.
Lee's group explore the probiotic roles of C.B in regulating CRC. They found that C.B inhibits colon cancer cell progression through inhibiting important cell functions: cell proliferation, stemness, apoptosis, patient derived organoid (PDO) growth, and metastases in CRC. Importantly, they demonstrated that C.B can promote MYC ubiquitination and accelerate the turnover rate of MYC, one of the most commonly activated oncoproteins in human cancer including CRC. Thus, C.B's negative impact on MYC expression accounts in part for its tumor suppressive impact.
Thymidylate synthase (TYMS), an enzyme has a central position in the pathway of DNA synthesis, is an important target for 5-fluorouracil (5-FU) chemotherapy. 5-FU has been used for treating CRC cancer, but 5-FU has toxicity and usually causes the prevalent unresponsiveness in patients, resulting in limited clinical benefits. They demonstrated that MYC binds to the promoter regions of TYMS. Importantly, MYC's binding to this promoter region was attenuated after C.B treatment. Significantly, C.B potentiates 5-FU treatment in cancer model. These data indicate that C.B can improve the therapeutic efficacy of 5-FU chemotherapy in CRC through interfering MYC-TYMS regulation.
Immunotherapy with immune-checkpoint inhibitors (ICIs) has revolutionized the treatment of various types of cancer. However, the majority of patients, including CRC, receiving these ICI therapies still have limited clinical benefit. There is an urgent need to boost the efficacy of ICI immune therapy. Lee’s group showed that C.B can obviously boost the response in immunotherapy (anti-PD1 therapy) of CRC by downregulating MYC, increasing CD8/CD4 T cell infiltration and Granzyme B in cancer model.
Clinical application of probiotics in CRC
In summary, these studies uncover C.B as a potential anti-cancer agent regulating important aspects of drug resistance and immune surveillance escape during CRC treatment. They demonstrate that C.B reprograms the proliferation, migration, stemness, and tumor growth in CRC by regulating pivotal signal molecules including MYC. Destabilization of MYC by C.B supplementation suppresses cancer cell proliferation/metastasis, sensitizes 5-FU treatment, and boosts responsiveness of anti-PD1 therapy. Thus, this study provides insight into the tumorigenesis modulating mechanisms of C.B in boosting chemo/immune therapies. Harnessing the anti-cancer effects of C.B could facilitate the development of more therapeutic designs to manage CRC.
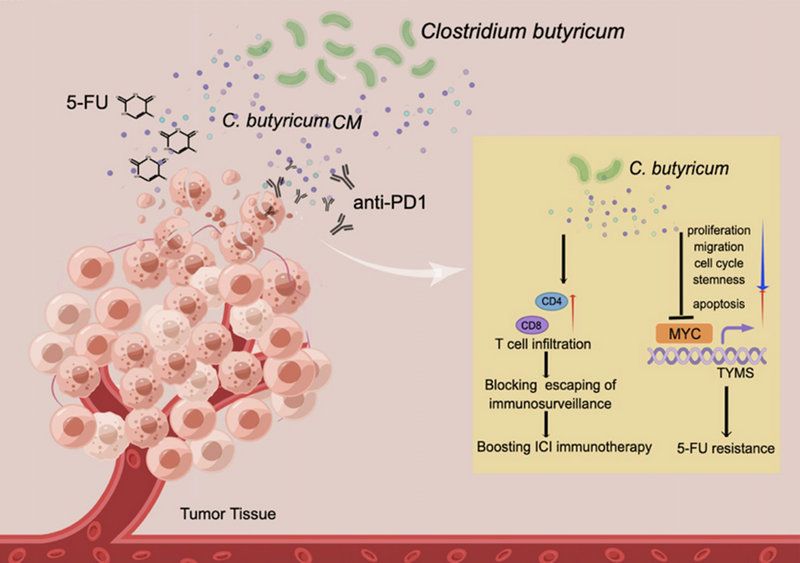
Schematic summary of C.B's role in modulating 5-FU drug resistance and boosting anti-PD1 immunotherapy.
Link to the article: https://www.tandfonline.com/doi/full/10.1080/19490976.2023.2186114



