Thyroid associated ophthalmopathy (TAO) is an autoimmune condition affecting the orbit and ocular adnexa that may lead to vision loss. Treatment for TAO remains challenging because of the poor understanding of its pathogenesis. On July 26, 2022, Prof. Wenru Su's group from Zhongshan Ophthalmic Center of Sun Yat-sen University recently published their results in Cell Reports Medicine, which revealed the immune microenvironment at the single-cell level in orbital tissues of thyroid associated ophthalmopathy, and provided a new direction for exploring potential therapeutic targets of TAO.
TAO is an organ-specific autoimmune disease characterized by infiltrative degeneration of posterior eyeball and periorbital tissues. It is one of the most common extra-thyroid manifestations of thyroid diseases. TAO usually presents with protruding eyes, eyelid retraction, diplopia, and even endangers vision and leads to blindness in severe cases. Treatment for TAO remains challenging because of the poor understanding of its pathogenesis. Therefore, deep analysis of the immune microenvironment in orbital connective tissue of TAO is expected to further clarify the pathogenesis and potential therapeutic targets of TAO.
In this work, researchers utilized single-cell RNA sequencing to perform transcriptome analysis of orbital connective tissue from TAO patients undergoing orbital decompression surgery versus normal controls undergoing blepharoplasty. Researchers developed a comprehensive transcriptome atlas of TAO by cell subsets identifying, differential expressed genes and cell-cell interaction analysis (FIG. 1).
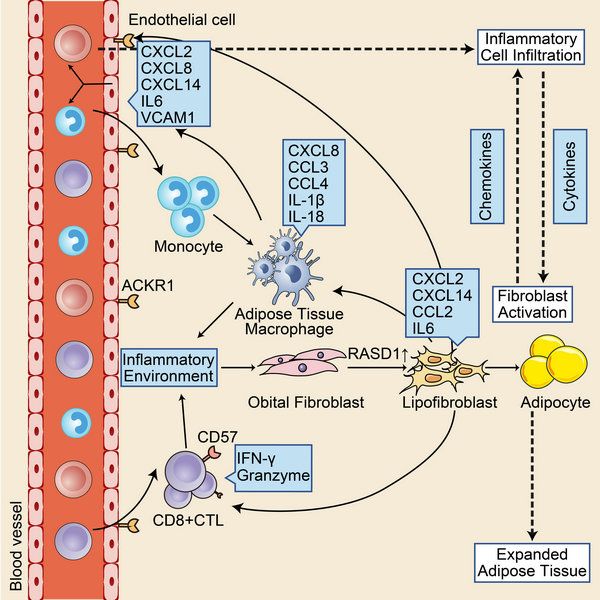
FIG.1 immune microenvironment in orbital connective tissue of TAO
The results showed that cells in orbital connective tissue could be clustered into fibroblasts, endothelial cells, pericytes, B cells, T cells, neutrophils and monocytes. Compared with the healthy control, orbital tissues of TAO showed obvious inflammatory cell infiltration, and the proportion of immune cells in TAO increased. (FIG. 2)
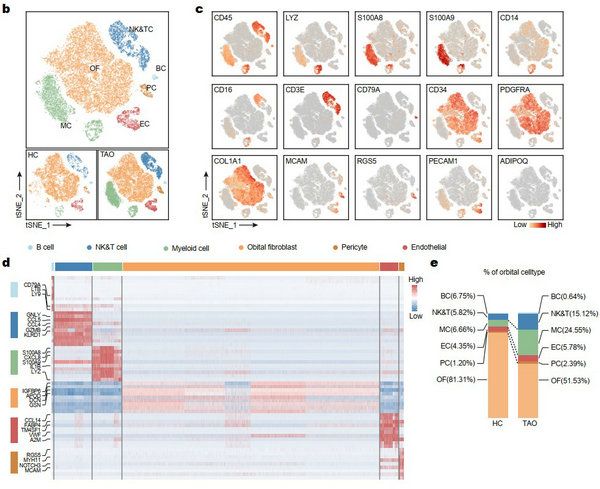
FIG. 2 cell clusters in orbital connective tissue
Orbital fibroblast is identified as the core of TAO pathogenesis. The analysis of fibroblast revealed that the RASD1 expressing fibroblast subsets, known as lipofibroblasts (LPF), expressed adipogenesis- or lipid accumulation-related transcription factors such as PPARG, CABPB, and PLIN2, and also highly expressed chemokines and inflammatory genes such as IL6 and CCL2. These results indicated that this cell cluster was highly involved in the pathogenesis of TAO. Fibroblasts attracted inflammatory cells to infiltrate into orbital tissue through chemokines. They also have the potential to differentiate into adipocytes. Inflammatory cell infiltration and secretion of inflammatory factors are the main causes of fibroblast activation. Pseudotime analysis also found that the fibroblast differentiation toward LPF increased in TAO. (FIG. 3)
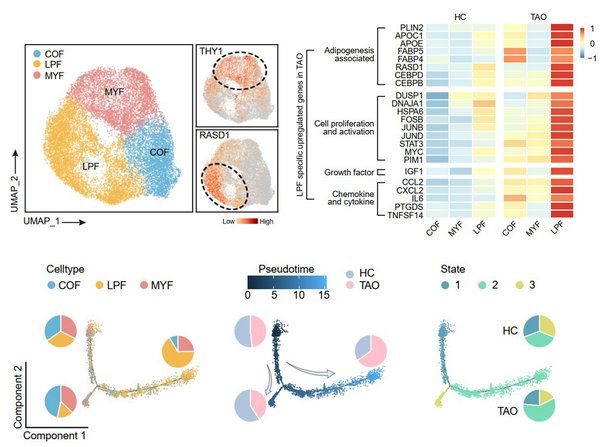
FIG. 3 analysis of orbital fibroblast
Analysis of endothelial cells (ECs) showed that atypical chemokine receptor 1+ (ACKR1+) ECs were significantly increased in TAO, and highly expressed adhesion molecules, selectin and chemokines. Further functional analysis showed that ACKR1+ ECs played an important role in the process of immune cells infiltration. Therefore, the researchers proposed that ACKR1+ ECs may also be involved in the pathogenesis of TAO. (FIG. 4)
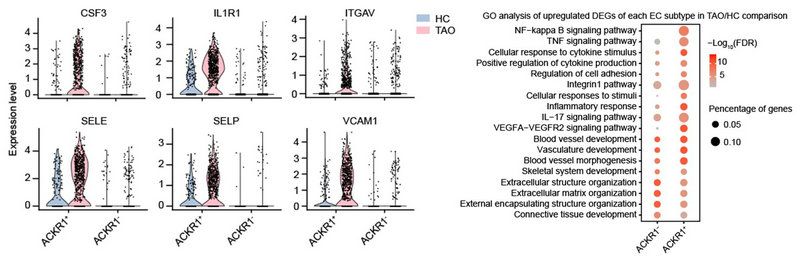
FIG. 4 analysis of endothelial cells
Interferon can stimulate the activation of fibroblasts, secrete chemokines and inflammatory factors, further proliferate and differentiate, and lead to the pathological changes of TAO. In addition to the source of CD4+Th cells in previous study, interferon in orbital is also a source of CD8+ cytotoxic T cells (CTL). CD8+CTL showed terminal differentiation phenotype, high expression of CD57 and CX3CR1, and high expression of granzyme and perforin. Terminally differentiated T cells can still maintain their function for a long time. Therefore, CD8+ CTL may be one of the persistent sources of interferon in TAO.
In addition, adipose tissue macrophage (ATM) was found in the orbital tissue. The typical manifestations of ATM are the expression of both type ⅱ macrophage markers such as CD163 and CD206, and the inflammatory secretion function of type ⅰ macrophages (IL-1β, CCL2, CCL3, etc.), as well the high expression of fatty acid transporter CD36. ATM was first discovered in adipose tissue of patients with diabetes and obesity. Its inflammatory secretory function is one of the causes of adipose tissue inflammation and insulin resistance. Therefore, ATM may also be involved in the inflammatory response of orbital adipose tissue in TAO. (FIG. 5)
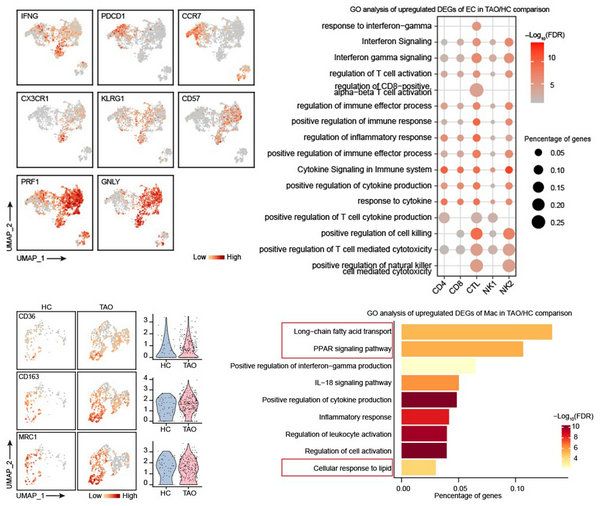
FIG. 5 analysis of immune cells
In conclusion, this work extends our understanding of the orbital immune microenvironment of TAO. It provides a new direction for exploring the potential therapeutic targets of TAO.
This research was online published in Cell Reports Medicine on July 26, 2022, with Professor Wenru Su from Zhongshan Ophthalmic Center of Sun Yat-sen University and Professor Xianggui Wang from Xiangya Hospital of Central South University as co-corresponding authors. Li Zhaohuai, a graduate student from Sun Yat-sen Ophthalmology Center of Sun Yat-sen University, Professor Mei Wang from Sun Yat-sen Memorial Hospital of Sun Yat-sen University, Jia Tan from Xiangya Hospital of Central South University, Lei Zhu, a graduate student from Sun Yat-sen Ophthalmology Center of Sun Yat-sen University, Dr. Peng Zeng from Sun Yat-sen Memorial Hospital of Sun Yat-sen University, and Xiaozhen Chen from Xiangya Hospital of Central South University were co-first authors. Zhongshan Ophthalmology Center and State Key Laboratory of Ophthalmology of Sun Yat-sen University were the first units. The research was supported by the National Science Fund for Excellent Young Scholars of China.
Link to the paper: https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(22)00235-X



