Source: The Sixth Affiliated Hospital
Edited by: Zheng Longfei, Wang Dongmei
Recently, an epigenetic technology ‘CD8+ MeTIL’ was developed by the group led by Drs Yanxin Luo and Huichuan Yu at the Sixth Affiliated Hospital of Sun Yat-sen University. This work has been published in the
Journal of Immunotherapy of Cancer (JITC, IF=13.7). This technology can accurately and quantitatively assess the abundance of infiltration CD8+ T cells in the tumor microenvironment. It provides a useful and novel tool for future research on tumor microenvironment and has important value of biomarker in tumor immunotherapy.
This study was initiated and conducted at the Sixth Affiliated Hospital of Sun Yat-sen University, and the teams in University of Washington Medical Center, Mayo Clinic, University of Toronto and University of Melbourne also participated in the project, worked with Dr Luo’s group, and critically contributed to this work. The paper is entitled "DNA methylation-based signature of CD8+ tumor-infiltrating lymphocytes enables evaluation of immune response and prognosis in colorectal cancer". Drs Huichuan Yu and Yanxin Luo are the corresponding authors of this article, and Drs Qi Zou, Xiaolin Wang and Donglin Ren are the co-first authors.
Colorectal cancer is one of the leading causes of cancer-related death worldwide. The current treatment strategies include surgical resection, radiotherapy, chemotherapy, targeted therapy, and emerging immunotherapies. However, the response to these treatments is well known to vary among individual patients. Currently, immune checkpoint inhibitors, such as PD-1, have reshaped cancer treatment. Unfortunately, many patients would not get survival benefit or develop resistance during the treatment.
The abundance of CD8+ T cells and their functional status in the tumor microenvironment have a critical impact on the outcome of therapeutics. Understanding the atlas of CD8+ T cells in the tumor microenvironment can predict the immune response of tumors during treatment, which has clinical significance in decision making.
DNA methylation is a chemical modification of DNA, which can produce heritable changes without changing the DNA sequence. The dynamic changes of DNA methylation play a key role in the differentiation, maturation and activation of CD8+ T cells. Moreover, DNA methylation is tissue-specific. DNA methylation profile varies in the cells from different tissues and organs, and thus it has the potential to be a marker to classify different types of cells in the mixed tissue samples.
In this study, the investigators generated a CD8+ T cell-specific DNA methylation signature and constructed a CD8+ MeTIL score to assess CD8+ TILs in CRC. The team has been focusing on DNA methylation in the past decade. In the previous work, the team developed a qPCR-based quantitative assay of genomic methylation at single-base resolution (QASM) (Clinical Chemistry 2019). With this technology, the detection of CD8+ MeTIL and the quantitative evaluation of tumor-infiltrating immune cells could be well achieved through three fast PCR reactions. The technical process is easy to be standardized with cheap and fast procedures that could be well replicated. The results in multiple clinical centers showed that CD8+ MeTIL predicted the outcome after immune checkpoint blocker therapy. This finding showed the potential of this promising tool in clinical translation. The team has applied for an invention patent: DNA methylation detection kit for evaluating tissue immune response and diagnostic prognosis (202011176816.1).
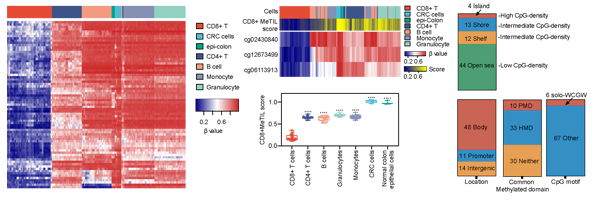
Figure 1. CD8+ T cell-specific methylation signature
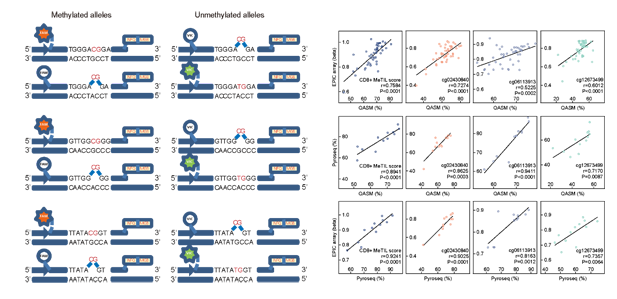
Figure 2. Principle and workflow of determining CD8 + MeTIL with qPCR-based QASM assay
CD8+ MeTIL is a digital cell classifier that can accurately quantify CD8+ T cells in the tumor microenvironment at the epigenetic level. The integrating analysis with other omics data would help to understand the role of epigenetic regulation in the differentiation, activation and anti-tumor immunity of CD8+ T cells. CD8+ MeTIL could play a role of surrogate biomarker to provide valuable information in guiding treatment such as immunotherapy in clinical settings.
Access to the original article:
https://jitc.bmj.com/content/9/9/e002671



