Source: School of Pharmaceutical Sciences
Written by: School of Pharmaceutical Sciences
Edited by: Wang Dongmei
J. Am. Soc. Chem. online published on 8th February, 2017 a research article entitled “Experimental and Theoretical Studies on Rhodium-Catalyzed Coupling of Benzamides with 2,2-Difluorovinyl Tosylate: Diverse Synthesis of Fluorinated Heterocycles” from Honggen Wang’s group at Sun Yat-sen University and Xingwei Li’s group at Dalian Institute of Chemical Physics, CAS. The article described the efficient syntheses of several types of mono- and di-fluorinated heterocycles via Rh(III)-catalyzed C-H annulation reactions with easily accessible 2,2-difluorovinyl tosylate. Prof. Honggen Wang and Prof. Xingwei Li are the corresponding authors. Dr. Jia-Qiang Wu, Shang-Shi Zhang and Dr. Hui Gao are co-first authors. (J. Am. Chem. Soc., 2017, 139, 3537–3545. http://pubs.acs.org/doi/abs/10.1021/jacs.7b00118)
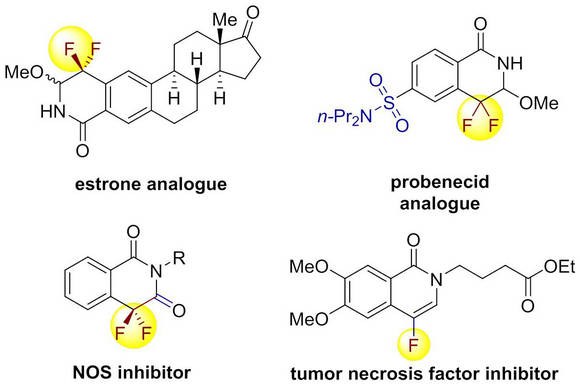
Organofluorine compounds and heterocyclic systems have had a profound impact in modern drug development. The introduction of fluorine or fluorine-containing structural motifs into small molecules often brings about desirable properties in pharmaceuticals and agrochemicals. However, due to the unique chemical reactivity of fluorine atom, the rapid and efficient construction of fluorinated heterocycles has long been a formidable challenge faced by medicinal and organic chemists. To tackle this problem, the research team evoked a direct C-H annulation strategy by harnessing the reactivity of fluorinated alkene.
By using 2,2-difluorovinyl tosylate as a novel coupling partner, the reaction of N-OMe benzamide under the catalysis of Rh(III) delivered a monofluorinated alkene, which was treated with acid to give the 4-fluoroisoquinolin-1(2H)-one product in one-pot. With N-OPiv benzamide as substrate, the direct formal [4+2] cyclization occurred to provide the gem-difluorinated dihydroisoquinolin-1(2H)-one in good efficiency.Systematic mechanistic studies by using experimental and theoretical methods were conducted and a new coplanar b-F elimination mechanism was proposed. The synthetic utility of the reaction was evidenced by the successful elaboration of two drugs (estrone and probenecid) and rapid assembly of two bio-active compounds (NOS inhibitor and tumor necrosis factor inhibitor), demonstrating its application potential in the field of drug discovery.
Prof. Honggen Wang’s research group has an ongoing interest in exploring the new reactivities of pre-functionalized unsaturated carbon carbon bond for the sake of construction of value-added molecules. Previously, they have successfully developed the synthesis of a-boron carbonyl compounds via oxidative difunctionalization of borylated alkenes. The elaboration of the products allowed the efficient construction of borylated heterocycles (Angew. Chem. Int. Ed.2016, 55, 10069-10073).



