A collaborative team led by Professor Chaoyun Pan from the Zhongshan School of Medicine has identified a neuro-immune-metabolic axis that drives chemoresistance, suggesting fluoxetine as a novel chemosensitizer.
Ovarian cancer remains one of the most formidable challenges in women's health, claiming approximately 200,000 lives annually. While initial responses to cytoreductive surgery and platinum-based chemotherapy are often positive, nearly 70% of patients experience recurrence within three years. This high mortality rate is largely driven by chemotherapy resistance, particularly the enhanced ability of tumor cells to repair their own DNA through homologous recombination (HR).
Recently, a multi-institutional team led by Professor Chaoyun Pan from the Department of Biochemistry at SYSU's Zhongshan School of Medicine, in collaboration with Professor Junxiu Liu from the First Affiliated Hospital of SYSU, and Professor Qinglei Gao from Tongji Hospital of Huazhong University of Science & Technology's Tongji Medical College, published a groundbreaking study in the prestigious journal Cell Metabolism. Titled "Serotonin-licensed macrophages potentiate chemoresistance via inositol metabolic crosstalk in ovarian cancer," the study reveals how peripheral serotonin (5-HT) fuels chemotherapy resistance and identifies a common antidepressant as a potential solution.
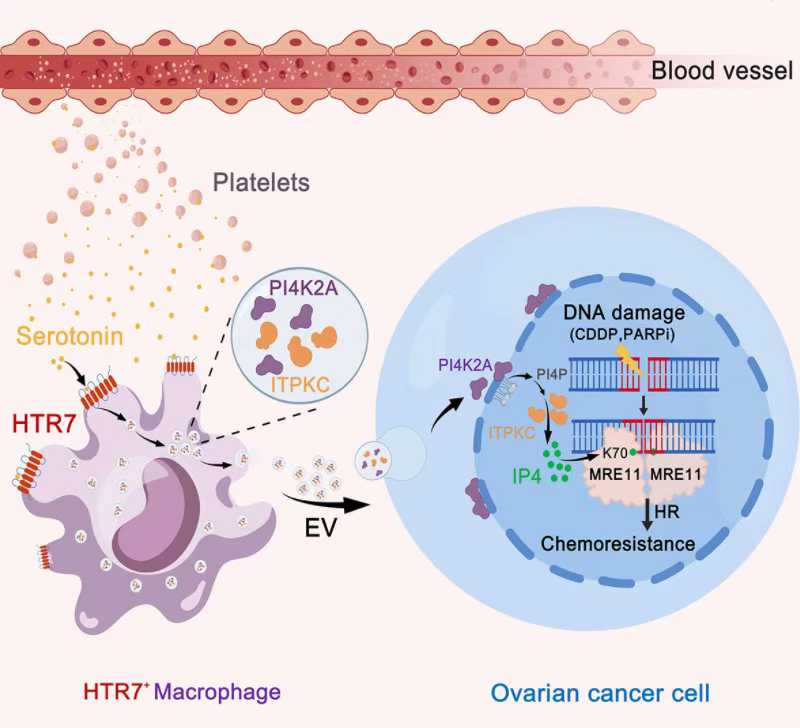
The researchers utilized a high-grade serous ovarian cancer mouse model that mimics the clinical evolution of drug resistance. Through a combination of single-cell sequencing and metabolomics, they discovered that serotonin released by platelets infiltrating the tumor acts on a specific subpopulation of macrophages expressing the HTR7 receptor. This interaction activates a signaling axis that stimulates these macrophages to secrete a large volume of extracellular vesicles. These vesicles, in turn, deliver key enzymes of inositol metabolism—specifically PI4K2A and ITPKC—directly into the tumor cells.
This metabolic transfer leads to a significant accumulation of inositol-1,3,4,5-tetrakisphosphate (IP4) within the tumor cell nucleus. The team found that nuclear IP4 directly binds to the protein MRE11, significantly enhancing its affinity for DNA. This interaction effectively "supercharges" the tumor's DNA repair capacity, allowing it to survive the damage typically inflicted by platinum-based drugs and PARP inhibitors.
Beyond identifying the problem, the study proposes a practical clinical solution. The researchers discovered that fluoxetine, a widely prescribed SSRI antidepressant, can effectively disrupt this resistance cycle by lowering peripheral serotonin levels. In various mouse and patient-derived tumor xenograft (PDX) models, the combination of fluoxetine with standard chemotherapy inhibited the activation of HTR7+ macrophages and reduced the tumor's DNA repair capability. This combined regimen significantly delayed tumor recurrence and reduced tumor burden without introducing additional toxic side effects.
Working Mechanism Diagram
This study marks the first time science has identified a regulatory axis involving "neurotransmitter-immune cell-metabolic crosstalk-tumor DNA repair." By demonstrating that a common antidepressant can act as a chemosensitizer, the research offers a promising, cost-effective strategy for maintenance therapy. The team is now actively moving toward clinical trials to verify the efficacy and safety of the "chemotherapy + SSRI" regimen for ovarian cancer patients.
The research was a collaborative effort involving the Advanced Technology Research Institute of Zhongshan School of Medicine - the First Affiliated Hospital of SYSU, the Metabolomics Research Center and the Laboratory Animal Center of Sun Yat-sen University, and the National Clinical Research Center for Gynecological and Obstetric Diseases at Tongji Hospital of Huazhong University of Science & Technology's Tongji Medical College.
Link to the paper: https://doi.org/10.1016/j.cmet.2025.11.011
Source: Zhongshan School of Medicine



