The research team led by Jie Xu, a researcher from the First Affiliated Hospital of Sun Yat-sen University published an article titled “NINJ1 regulates plasma membrane fragility under mechanical strain” in Nature on June 9. In collaboration with researchers from Rutgers University, the team developed the world's first high-throughput mechanical tension stimulation system and employed it to identify a key protein, NINJ1, which is found to play a critical role in cell death mechanisms. This discovery offers novel therapeutic perspectives for the treatment of tissue damage, excessive inflammatory responses, and autoimmune diseases.

The research findings are published in the prestigious international journal Nature.
The cell membrane serves as a critical guardian of cellular homeostasis, maintaining stability between intracellular and extracellular environments. However, when subjected to excessive mechanical stress, this protective barrier can rupture, releasing intracellular components—including DNA and damage-associated molecular patterns (DAMPs)—into the extracellular space. Such leakage triggers potent inflammatory cascades in neighboring cells. For decades, a fundamental question has persisted in medical research: What molecular mechanism determines whether mechanical force induces catastrophic membrane failure?
However, existing cell stretching systems on the market can only conduct a few experiments at a time, far from meeting the need for conducting three to four hundred experiments simultaneously. Therefore, Professor Xu Jie’s team decided to independently develop a new device to advance scientific progress. After nearly two years of work and five iterations, the team finally developed a prototype capable of applying uniform mechanical tension to large-scale cell populations — the high-throughput mechanical tension stimulation device.
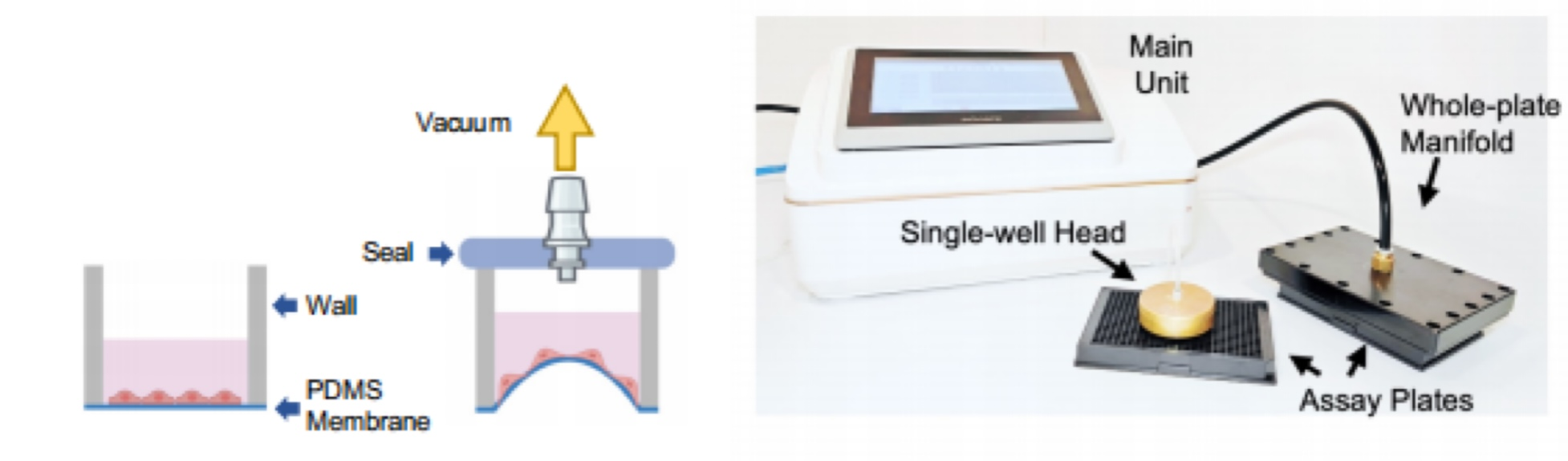
The high-throughput mechanical tension stimulation device
Using this device, the team systematically screened thousands of human multi-pass transmembrane proteins and ultimately discovered that NINJ1 is a critical regulator for mechanical strain-induced plasma membrane rupture (PMR).
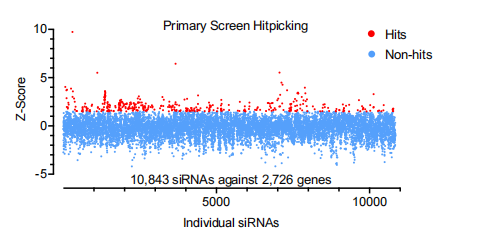
Schematic diagram of the research findings
Clinically, NINJ1 could become a novel target for modulating tissue damage, excessive inflammatory responses, and even autoimmune diseases. For example, in conditions such as lung injury, sepsis, or the tumor microenvironment, using small molecules to limit NINJ1 activity may help reduce tissue damage and effectively suppress the inflammatory storm in septic shock.

Researcher Jie Xu (left) guides team member in the experiment.
This research was funded by the National Natural Science Foundation of China and the Guangdong Provincial Department of Science and Technology. The Institute of Precision Medicine at the First Affiliated Hospital of Sun Yat-sen University provided critical platform support for the research. The high-throughput mechanical stimulation platform developed in this study can be widely applied in the future for screening other mechanically sensitive genes, as well as for drug screening and development related to mechanical force-associated diseases.
Link to the paper:
http://doi.org/10.1038/s41586-025-09222-5



